Abstract
Introduction: The investigational drug GFH009 is a potent and highly selective small molecule inhibitor of cyclin-dependent kinase 9 (CDK9). Dysregulation of the CDK9 pathway has been observed in acute myeloid leukemia (AML) and other hematologic malignancies, thus rendering it an attractive target for cancer therapeutics. GFH009 binds to CDK9, inhibiting kinase activity and blocking RNA polymerase II-mediated transcription maturation, while showing only weak effects on other CDK family members, kinomes, and G-protein coupled receptors. This action inhibits expression of cell growth-promoting molecular targets downstream of CDK9, such as anti-apoptotic proteins. GFH009 shows strong potential as a treatment for hematologic malignancies such as lymphoma, small lymphocytic lymphoma, acute myeloid leukemia, and chronic lymphocytic leukemia.
Methods: GFH009 activity was assessed in human hematologic malignancy cell lines via 24-hour in vitro anti-proliferative assays. Cellular apoptosis rates were observed for GFH009 in assorted human hematologic malignancy lines. Cells were treated with GFH009 at a range of doses for 4 hours, and protein levels of molecular products downstream of CDK9 were detected in human AML cell line MV-4-11 cells via Western blot analysis. The reference compound enitociclib (BAY 1251152) was used for experimental validation.
In vivo studies were conducted in MV-4-11 xenograft mice treated with GFH009 at a range of doses on a weekly or twice weekly schedule and compared to ENI. Tumor volume inhibition and body weight change were measured. Percent survival was measured through day-100 post-dose in an orthotopic MV-4-11 survival model. In vivo protein expression was assessed via Western blot post-treatment with GFH009.
Results: Treatment with GFH009 showed anti-proliferative activity in hematologic malignancy cell lines and induced apoptosis at a higher rate than both placebo and enitociclib. Western blot analysis showed significant reduction of Mcl-1, an anti-apoptotic protein, and the proto-oncogene products c-Myc, and p-Rpb1 carboxyl-terminal domain (CTD) Ser2, accompanied by induced cleaved caspase-3 following treatment with GFH009 at concentrations >0.1µM.
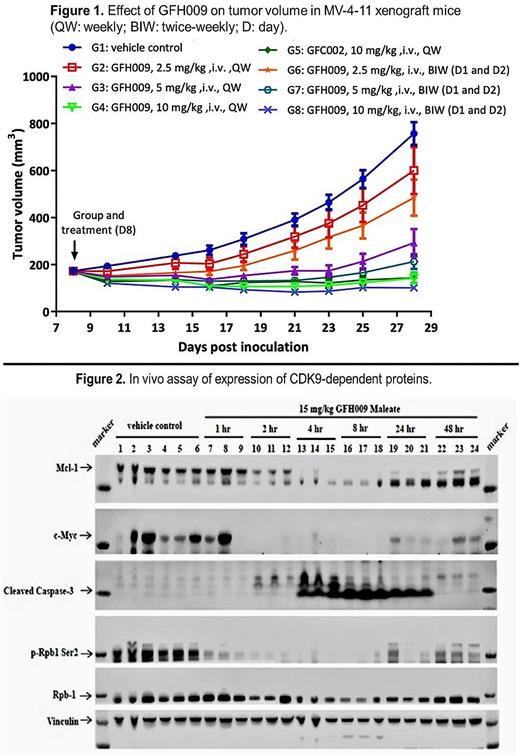
In MV-4-11 xenograft mouse models, GFH009 inhibited tumor growth as measured by total tumor volume (TV) at all doses compared with the control group. GFH009 inhibited tumor growth dose dependently at doses of 2.5 mg/kg. Notably, GFH009 at a dose of 10.0 mg/kg doses resulted in consistent TV reduction through day 29. More frequent dosing (twice a week vs once a week) showed greater inhibition of tumor growth in all doses tested (Figure 1). Body weight (BW) remained stable in all GFH009 treatment groups, and relative change in BW was within ±5% through day 28 post-inoculation. Treatment with GFH009 at 10 mg/kg twice weekly significantly prolonged survival time in MV-4-11 mice, while a 50% event-free survival rate was observed by day 82 post-treatment, compared to day 52 in the control group (a 57.7% longer interval). In agreement with in vitro studies, Western blot analysis of MV-4-11 xenografts indicated that GFH009 reduced the expression of Mcl-1 and c-Myc proteins in vivo (Figure 2).
Conclusions: Although several cytotoxic molecules that inhibit CDK9 are in the therapeutic pipeline, many show cross-reactivity to other CDK family members, leading to toxicity and tolerability issues. GFH009 is a potent and highly selective CDK9 inhibitor with the ability to reduce expression of downstream oncogenes required for rapid cellular division and protein expression through specific, short-lived inhibition of CDK9. We postulate that this mechanism drives GFH009's inhibition of cellular division, as tumor stabilization and shrinkage appear to be dose-dependent. Mcl-1 and c-Myc, both of which play crucial roles in protecting cells from apoptosis, showed reduced expression following treatment both in vitro and in vivo. This depletion via GFH009 inhibition of CDK9 likely deprives oncogene-addicted cancer cells of crucial survival signals, leading to senescence and death. These data suggest that GFH009 depletes anti-apoptotic molecular products downstream of CDK9 and merits further investigation as a potential treatment for hematologic cancers in humans.
Disclosures
Zhou:GenFleet Therapeutics: Current Employment, Patents & Royalties: 9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1. Tang:GenFleet Therapeutics: Current Employment, Patents & Royalties: 9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1. Le:GenFleet Therapeutics: Current Employment, Patents & Royalties: "9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1". Ge:GenFleet Therapeutics: Current Employment, Patents & Royalties: "9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1". Cicic:Sellas: Current Employment. Xie:GenFleet Therapeutics: Current Employment, Patents & Royalties: 9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1. Ren:GenFleet Therapeutics: Current Employment, Patents & Royalties: "9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1". Lan:GenFleet Therapeutics: Current Employment, Patents & Royalties: "9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1". Lu:GenFleet Therapeutics: Current Employment, Patents & Royalties: "9 patents issued: US10,952,999B2 EP3613737B1 JP6866967 KR10-2309986B1 AU2018253655 CA3059622C RU2738654C CN108727363B MO J/004377 1 patent pending: WO2020244612A1".
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal